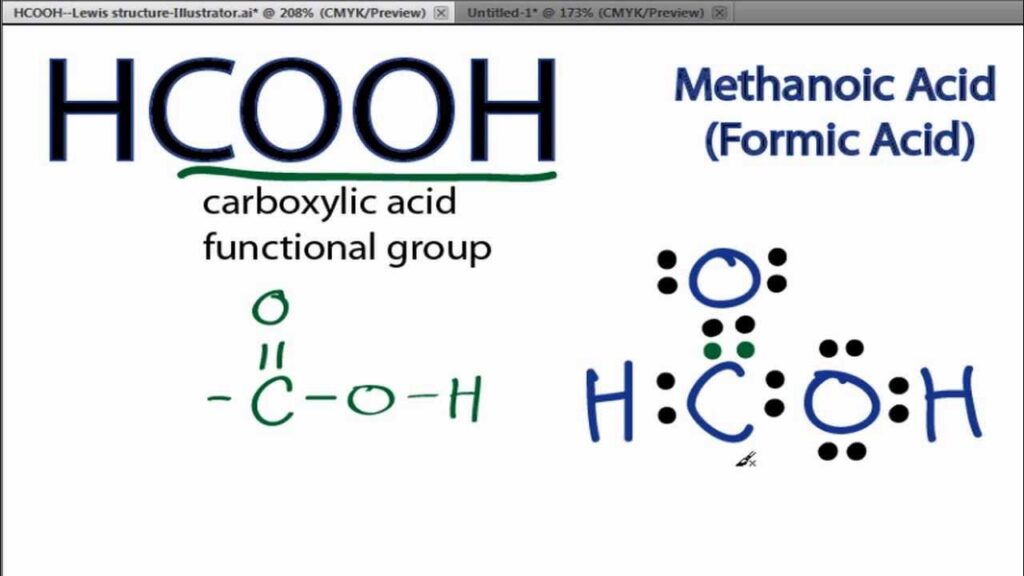
Introduction to Hcooch Ch2 H20
Nature is a complex web of interactions where every element plays a vital role. One fascinating compound that often flies under the radar is Hcooch Ch2 H20. This unique molecular structure isn’t just another chemical formula; it embodies the intricate dance between organic and inorganic components in our ecosystem. From supporting life to facilitating essential processes, understanding this compound opens up new insights into how nature operates.
Join us as we delve deeper into what Hcooch Ch2 H20 really is, its significance in nature, and how it connects with everyday occurrences around us. Whether you’re a science enthusiast or simply curious about the world we live in, there’s much to discover about this intriguing molecule!
What is Hcooch Ch2 H20?
Hcooch Ch2 H20 is a fascinating compound that combines elements of both organic and inorganic chemistry. At its core, this molecular structure consists of carbon, hydrogen, and oxygen atoms.
The formula represents a unique blend where the “Hcooch” part typically refers to an ester group. This group is known for its role in creating flavors and fragrances found in nature. The “Ch2” indicates the presence of additional hydrogen atoms bonded to carbon, enhancing reactivity.
Water (H2O) plays a critical role as well. It acts as a solvent and medium for many biological processes involving Hcooch Ch2 H20. Together, they interact dynamically within natural ecosystems.
This compound often showcases how simple molecules can work harmoniously to support life forms around us while contributing to various biochemical pathways essential for growth and development across different species.
The Role of Hcooch Ch2 H20 in Nature
Hcooch Ch2 H20 plays a vital role in various natural processes. It is involved in the formation of essential organic compounds that sustain life on Earth.
This compound facilitates energy transfer within ecosystems, supporting plant growth through photosynthesis. Plants absorb sunlight and convert it into chemical energy using Hcooch Ch2 H20. This process not only creates food for plants but also produces oxygen for other living beings.
Hcooch Ch2 H20 serves as a solvent, aiding in nutrient transport throughout organisms. It dissolves minerals and vitamins, making them accessible to cells for metabolic functions.
In aquatic environments, this compound helps maintain temperature balance and supports diverse habitats. The interaction between water molecules allows for unique ecological niches where countless species thrive. Understanding its role enriches our appreciation of nature’s complexity and interconnectedness.
How Does Hcooch Ch2 H20 Work Together in Nature?
Hcooch Ch2 H20 plays a vital role in various natural processes. It is often involved in biochemical reactions that support life on Earth. This compound acts as a building block for essential molecules.
In ecosystems, Hcooch Ch2 H20 facilitates nutrient transport. Plants absorb it through their roots, allowing them to thrive and produce oxygen via photosynthesis. This interaction supports diverse food chains.
The combination also aids in maintaining temperature balance within habitats. It regulates climate by absorbing heat during evaporation and releasing it during condensation. Such mechanisms are crucial for sustaining life forms.
Microorganisms rely on this compound, too, breaking it down for energy production. They contribute to soil health and nutrient recycling. Together, these functions illustrate how intertwined Hcooch Ch2 H20 is with the environment’s dynamics, showcasing its importance beyond mere chemical composition.
Applications of Hcooch Ch2 H20 in Everyday Life
Hcooch Ch2 H20 finds its way into various aspects of daily life, showcasing its versatility. In the realm of food science, it serves as a crucial ingredient in flavoring agents and preservatives. Its unique properties help maintain freshness.
In the beauty industry, this compound is often featured in skincare products. It acts as an effective moisturizer due to its ability to bind water molecules, keeping skin hydrated and healthy. Hcooch Ch2 H20 plays a role in agriculture. Farmers utilize it for soil enhancement and pest control. Its chemical structure aids in nutrient absorption for plants.
Household cleaning products also benefit from this compound’s effectiveness against grime and stains. It enhances cleaning power while being gentle on surfaces.
These applications illustrate how interconnected our lives are with compounds like Hcooch Ch2 H20, enriching experiences across diverse fields without us even realizing it.
Understanding the Chemistry behind Hcooch Ch2 H20
Understanding the chemistry behind Hcooch Ch2 H20 reveals a fascinating interplay of molecular components. At its core, this compound consists of carbon (C), hydrogen (H), and oxygen (O) atoms, each contributing to its unique properties.
The presence of the hydroxyl group in Hcooch adds polar characteristics. This polarity plays a crucial role in solubility and reactivity with other compounds. Meanwhile, the hydrocarbon chain provides hydrophobic attributes, allowing it to interact dynamically in various environments.
Hydrogen bonding is another important aspect. These interactions significantly influence boiling points and melting points. Such bonds also facilitate critical biological processes like enzyme activity.
Exploring these chemical interactions gives insight into how Hcooch Ch2 H20 behaves under different conditions. It highlights the delicate balance that sustains life across ecosystems while emphasizing its importance beyond mere composition.
Frequently Asked Questions About the Hcooch Ch2 H20
How is Methyl Formate (HCOOCH₃) Produced?
Methyl formate is typically synthesized by the reaction of methanol (CH₃OH) with carbon monoxide (CO) in the presence of a catalyst.
What Are the Industrial Uses of Methyl Formate?
Methyl formate is used in manufacturing pesticides, refrigerants, adhesives, and as a blowing agent in foam production. It is also a potential alternative fuel and is found in space as an interstellar molecule.
Is Methyl Formate (HCOOCH₃) Hazardous?
Yes, methyl formate is flammable, volatile, and can cause irritation when inhaled or exposed to skin. Proper handling and ventilation are necessary.
Does Methyl Formate Have Any Environmental Impact?
Methyl formate is biodegradable but can contribute to air pollution if released in large quantities. It is less harmful compared to traditional hydrocarbon solvents.
Can Methyl Formate Be Used as a Fuel?
Yes, researchers are exploring methyl formate as an alternative fuel due to its high energy density and lower environmental impact.
Conclusion and Future Implications
The relationship between Hcooch Ch2 H20 and nature is intricate and fascinating. This compound plays a vital role in various natural processes, from biological functions to environmental interactions. Understanding how these components work together opens up new possibilities for research and innovation.
As we continue to explore the applications of Hcooch Ch2 H20 in everyday life, its significance becomes increasingly clear. From improving agricultural practices to enhancing water purification methods, the potential benefits are vast.
Future implications could lead us toward sustainable solutions that harness the power of this unique compound. Ongoing studies may reveal even more about its capabilities and how it can contribute positively to our environment and daily lives.
Embracing the chemistry behind Hcooch Ch2 H20 not only enhances our understanding but also inspires future generations of scientists and innovators. The journey into this exciting field continues with endless opportunities for discovery.